Main activities and results 2016
WP 1 – Synthesis and characterization of various ionic liquid eutectic mixtures formulations suitable for applications in Sn alloys electrodeposition
A large range of formulations with and without dissolved metals salts have been prepared and characterized.
Figure 1 shows an example of the recorded electrical conductivity vs. temperature for choline chloride:ethylene glycol (1:2 molar ratio) and choline chloride:malonic acid (1:1 molar ratio) eutectic mixtures. Activation energy values between 10-20 kJ/mol have been calculated for the investigated temperature domain.
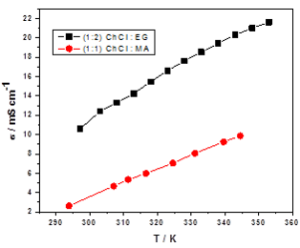
Figure 1 – Dependence of electrical conductivity vs. temperature for choline chloride:ethylene glycol (1:2 molar ratio) and choline chloride:malonic acid (1:1 molar ratio) eutectic mixtures
Figure 2 shows an example of the recorded electrical conductivity vs. temperature plots in the case of a complex system based on choline chloride:malonic acid (1:1 molar ratio) eutectic solvent (denoted ILM) containing Sn and Ag metallic salts as chlorides.
Activation energy values between 13-20 kJ/mol have been calculated for the investigated systems, lower as the AgCl concentration is higher.
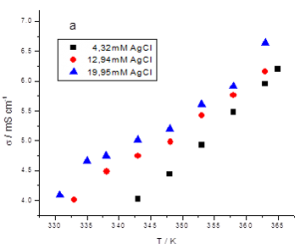
Figure 2 – Dependence of electrical conductivity vs. temperature in the case of a complex system based on choline chloride:malonic acid (1:1 molar ratio) eutectic solvent (denoted ILM) containing 0.5M SnCl2.2H2O and various AgCl concentrations
The addition of Cu salts in order to prepare electrolytes for Sn-Ag-Cu ternary alloy electrodeposition didn’t significantly affect the physical characteristics of the electrolytes. Figure 3 shows an example of the electrical conductivity vs. temperature plots for ternary systems in choline chloride:ethylene glycol (1:2 molar ratio) (denoted ILEG) and ILM.
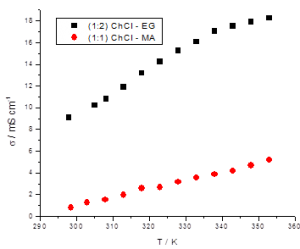
Figure 3 – Dependence of electrical conductivity vs. temperature in the case of a complex system based on ILM or ILEG eutectic solvents containing 0.5M SnCl2.2H2O + 12.94 mM AgCl + 3.67 mM CuCl2
WP 2 – Development and characterisation of Sn binary and ternary alloys coatings (Sn-Cu, Sn-Ni, Sn-Co, Sn-Ag, Sn-In, Sn-Ag-Cu, Sn-Ag-In, Sn-Cu-Ni)
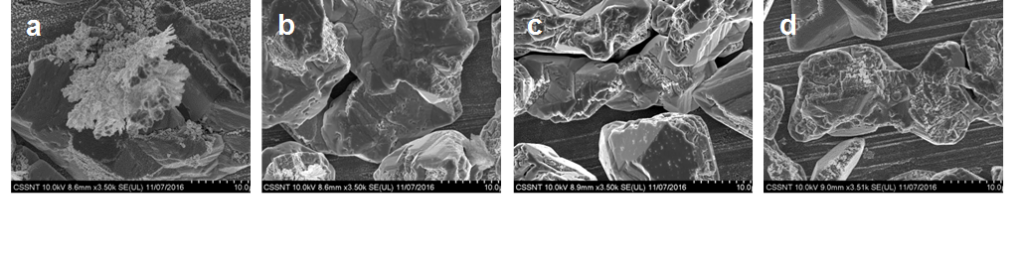
SEM micrographies for electrodeposited Sn-Ag alloy onto Cu substrate using electrolit ILEG+0.5M SnCl2+4.31mM AgCl at different applied current densities:(a) 2.67 mA/cm2; (b) 4.53 mA/cm2; (c) 8 mA/cm2; (d) 10.6 mA/cm2
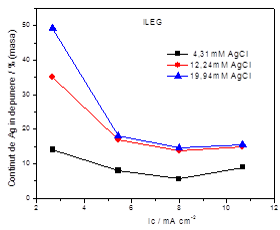
Dependence of Ag content within the alloy vs. the applied current density for an electrolyte containing 0.5M SnCl2 and various AgCl concentrations in ILEG
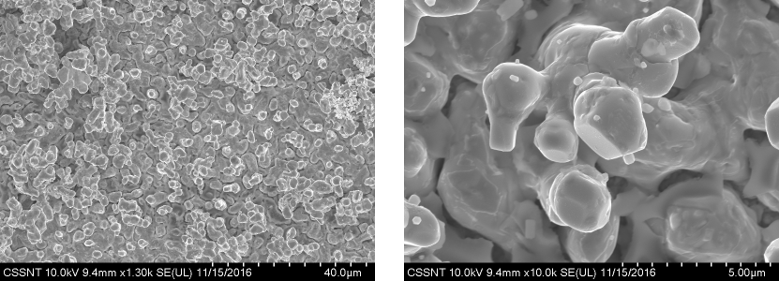
SEM micrographs for electrodeposited Sn-In (approx.33 wt.% In) alloy on Cu using ILEG eutectic mixture containing 50 mM InCl3 + 50 mM SnCl2 (2.5 mA/cm2, 30 min., 70oC)
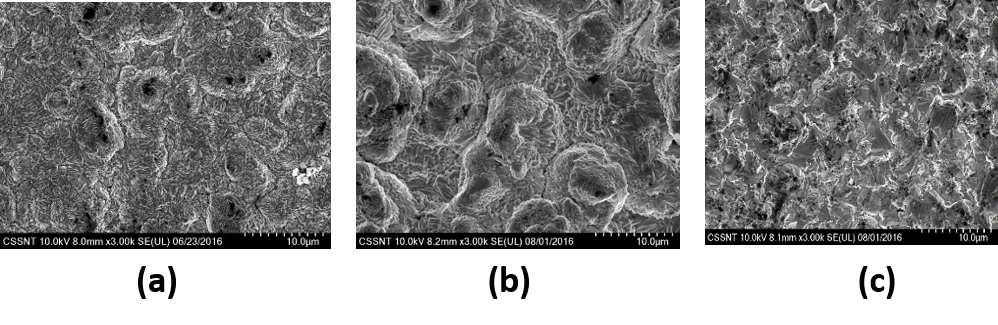
SEM micrographs for electrodeposited Sn-Co (15-17 wt.% Co) alloy on Cu from ILEG eutectic mixture containing 1M total concentration of metallic salts and various Co:Sn molar ratios in electrolyte: (a) 1:1; (b) 2:1; (c) 3:1

SEM micrographs for electrodeposited Sn-Ni composites with graphene (GO) under ultrasonic stirring (81 mA/cm2 , 500C, 30 min.)
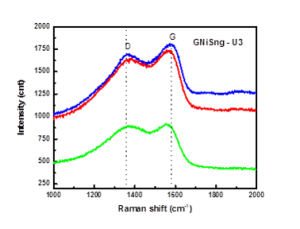
Raman spectra for Sn-Ni composites with graphene

SEM micrographs for electrodeposited Sn-Cu-Ag alloy using ILEG+500mM SnCl2+12.94mM AgCl+3.67 mM CuCl2 at different applied current densities: (a) 2.67 mA/cm2; (b) 5.4 mA/cm2; (c) 8 mA/cm2; (d) 10 mA/cm2