MAIN ACTIVITIES AND RESULTS – 2014
WP1 – Synthesis and characterization of various ionic liquid eutectic mixtures formulations suitable for applications in metals and alloys electrodeposition and anodic oxidation of valve metals
A large range of formulations with and without dissolved metals salts have been prepared and characterized.
Figure 1 shows an example of the recorded electrical conductivity vs. temperature for a system based on choline chloride:urea (1:2 molar ratio) eutectic mixture with various additions of citric acid in the range of 0.095M – 1.9M. Activation energy values between 26-40 kJ/mol have been calculated for the investigated temperature domain.
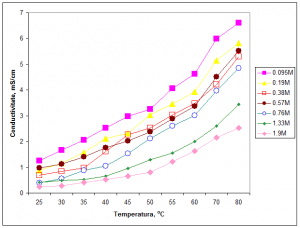
Figure 1 – Dependence of electrical conductivity vs. temperature for a system based on choline chloride:urea (1:2 molar ratio) eutectic mixture with various additions of citric acid
Figure 2 shows an example of Arrhenius plots (lns – 1/T coordinates) for a system containing 0.42M NiCl2.6H2O + 0.016M AHMo(ammonium heptamolybdate) in ILC ( 0.83M citric acid in choline chloride-urea 1:2), including activation energy determination.
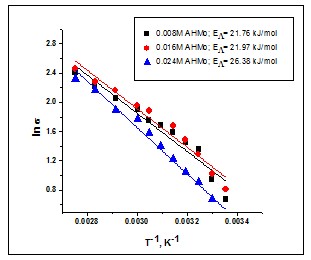
Fig.2. Arrhenius plots of conductivity vs. 1/T for Mo based ionic liquids: ILC-0.42M NiCl2.6H20-AHMo
Values of electrical conductivities for choline chloride based systems containing CoCl2 and CoCl2 +Mo(VI) ions are illustrated in Figure 3, as Arrhenius plots.
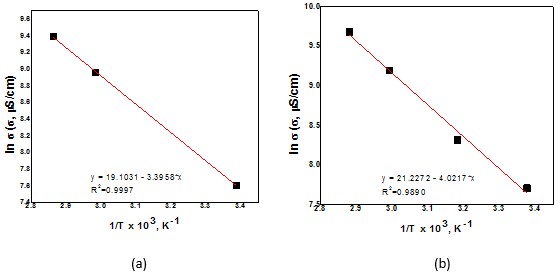
Fig.3 – Arrhenius plots (lns – 1/T coordinates) for systems containing:
(a) 0.50 M Co2+; (d) 0.039 M Mo(VI) + 0.50 M Co2+
WP2 – Development and characterization of corrosion resistant coatings (Cr, Ni and Co alloys with Mo and/or V, metal-CNT composites)
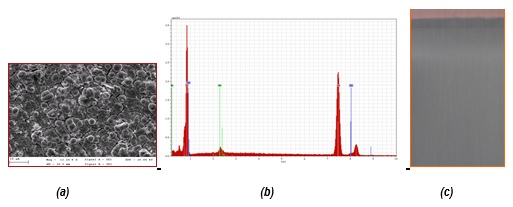
SEM micrography and EDX analysis (a, b) for Ni-Mo alloy coatings electrodeposited from ILC-NiMo system for 30 min., at 4.65 A/dm2 and (c) photograph of real Ni-Mo alloy coatings onto Cu substrate (i=18 mA/cm2, 50oC, 60 min.)
The evaluated deposition rate was in the range of 0.1 – 0.4 µm/min. for a current density domain between 20 mA/cm2 – 120 mA/cm2.
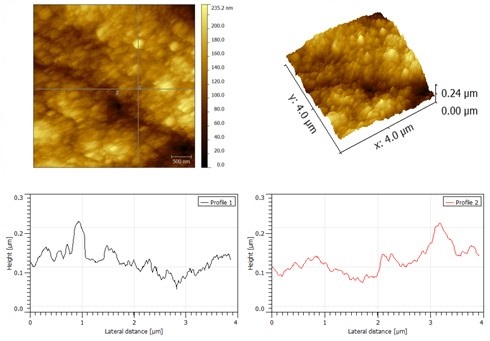
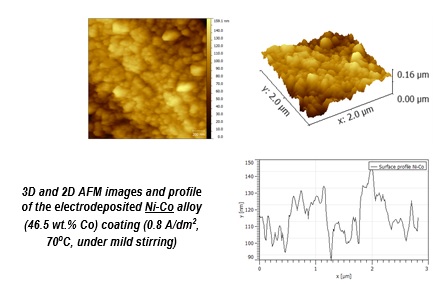
AFM micrographiesand profiles for Co-Mo alloy coatings electrodeposited from 0.039M Mo(VI) – 0.5M Co2+ in choline chloride: urea eutectic mixture, at 0.75 A/dm2, 95oC (4*4 μm)
WP 3 – Anodic oxide nanostructures on valve metals involving ionic liquids based electrolytes – electrochemical synthesis and characterization
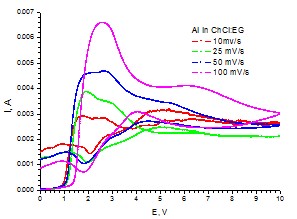
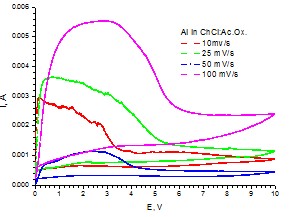
Cyclic voltammograms for Al working electrode (S=0.19 cm2) in choline chloride:ethylene glycol eutectic solvent at various scan rates (25oC, stationary conditions)
Cyclic voltammograms for Al working electrode (S=0.19 cm2) in choline chloride:oxalic acid eutectic solvent at various scan rates (25oC, stationary conditions)
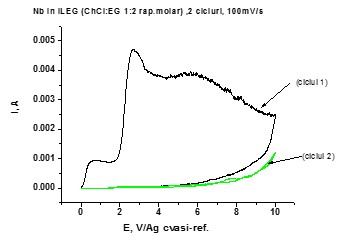
Cyclic voltammograms for Nb working electrode (S=0.19 cm2) in choline chloride:ethylene glycol eutectic solvent at 100mV/s, two scans (25oC, stationary conditions)
The preliminary experimental results regarding the anodic behavior of valve metals in choline chloride based ionic liquids systems suggest a relatively different as compared to the case of water based electrolytes.
The equilibrium between the metal anodic dissolution process and the anodic oxidation one facilitating the anodic oxide layer growth seems to be displaced towards the metal dissolution, probably due to the presence of Cl– ion within the eutectic mixture composition, mainly in the case of Al electrode.